News + Events
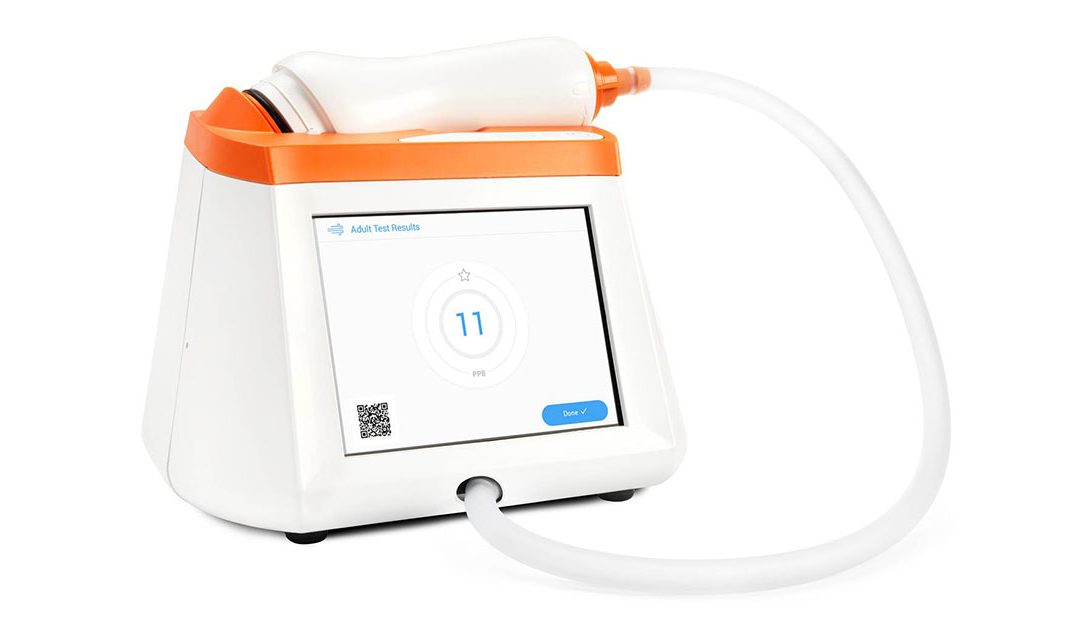
FDA Clears Fenom Pro 6-second Test Mode
Major Milestone for Expanded Access to FENO Testing in US Pleasanton, CA, June 22, 2023 — CAIRE Diagnostics is pleased to announce that the Fenom Pro® can now be upgraded to include a 6-second test mode available for ages 6 and up. The completed...

CAIRE Completes Acquisition of MGC Diagnostics Holdings, Inc.
Company Further Expands Clinical Healthcare Portfolio for Patients Facing Pulmonary Disease Ball Ground, GA (January 4, 2023) — CAIRE Inc., the leading global manufacturer of oxygen therapy and on-site generation systems, has finalized the...

CAIRE to Acquire MGC Diagnostics Holdings, Inc.
Portfolio Expands to Include Non-Invasive Cardiorespiratory Diagnostic Systems Ball Ground, GA, USA (November 29, 2022) — CAIRE Inc., a subsidiary of NGK SPARK PLUG CO., LTD., will acquire St. Paul, Minnesota-based MGC Diagnostics Holdings, Inc....
Subscribe to Diagnostics News + Events
Related Posts
FDA Clears Fenom Pro 6-second Test Mode
Major Milestone for Expanded Access to FENO Testing in US Pleasanton, CA, June 22, 2023 — CAIRE...
NGK SPARK PLUG CO., LTD. Implements Name Change
CAIRE® Branded As Part of Niterra Group Global oxygen equipment manufacturer CAIRE Inc.’s parent...
